Published on Chemistry-Methods!
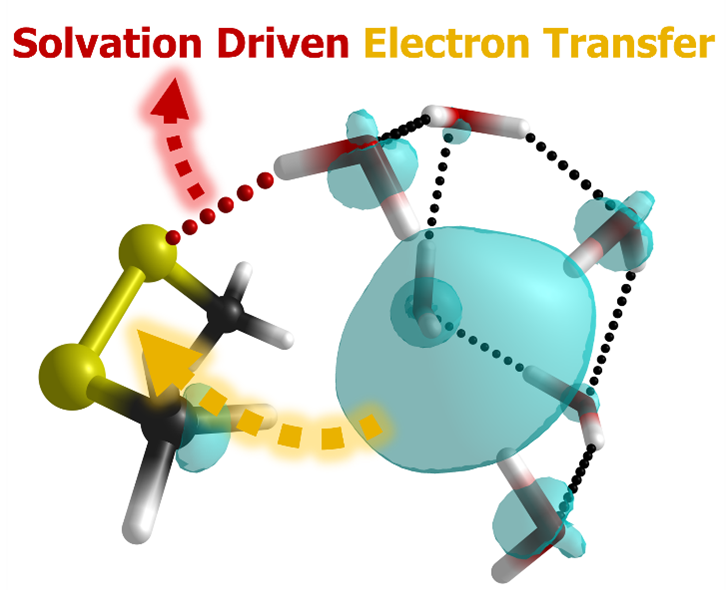
We explored the gas-phase ion chemistry of hydrated electron in the reaction with a prototypical organosulphur compounds, i.e. dimethyl disulphide. We showed that the overall reaction is gated by a critical S···H hydrogen bond, which enables a kinetically challenging electron transfer step. The overall exothermicity is not driven by electron transfer itself but by a large release of solvation energy that stabilizes the nascent radical anion of dimethyl disulphide. Following the electron transfer, the system undergoes a complex solvent reorganization driven by dynamic hydrogen bonding, where a relaxation of the initial hydrated electron cavity competes with the stabilization of the resulting radical anion. These findings suggest that insufficient solvation can either kinetically hinder the initial electron transfer or by subsequently failing to stabilize the anion, promote S-S bond cleavage.
